Clinical trials are a key component of research. Scientists use medical knowledge to ask and answer scientific or medical questions in areas such as medication, medical device, teaching concepts, or behavioral change. Researchers first test new therapies in labs and/or animals. Treatments that are safe are moved into clinical trials to move the drug or therapy toward approval by the FDA.
Commonly Used Terms
This list was developed to provide easy-to-understand definitions of commonly used terms in clinical trials. Commonly Used Clinical Terms
Types of Clinical Trials

Treatment
Test new treatments or devices, new combinations of drugs, or new approaches to surgery or radiation therapy. Treatment trials are often categorized by phases I through III.
Natural History
Provide information about how health and disease progress over time.
Prevention
Evaluate the effectiveness of ways to reduce the risk of developing a disease or preventing a disease from returning.
Diagnositic
Develop better tests or procedures to identify/diagnose a particular disease or condition.
Screening
Assess new ways of detecting disease earlier in healthy people.
Quality of Life (or Supportive Care)
Evaluate measures to improve comfort of and quality of life for people with chronic illnesses through better therapies or psychosocial interventions.
Clinical Trial Phases

MitoAction was recently a part of an FDA Listening Session with the Pyruvate Dehydrogenase Complex Deficiency (PDCD) Community. The purpose of this listening session was to build a stronger relationship between the FDA and the PDCD community so the FDA can get a better understanding of this community’s urgent medical needs. Click on the graphic for the full report.

MitoAction and the Barth SyndromeA rare, genetic disorder of lipid metabolism that primarily affects males. community need your voice to encourage the FDA to use the flexibility given to them by Congress to provide an equitable, fair, and appropriate review of a new drug application for elamipretide. If you have already taken the time to sign the petition, please consider going one step further by sending the FDA a letter by this Friday (December 22nd)! All you have to do is click on the link below, and the Barth Syndrome Foundation will walk you through writing and submitting your letter! It will only take 5 minutes!
Click HERE to write a quick letter to the FDA
Click HERE to read an article for more information on the situation
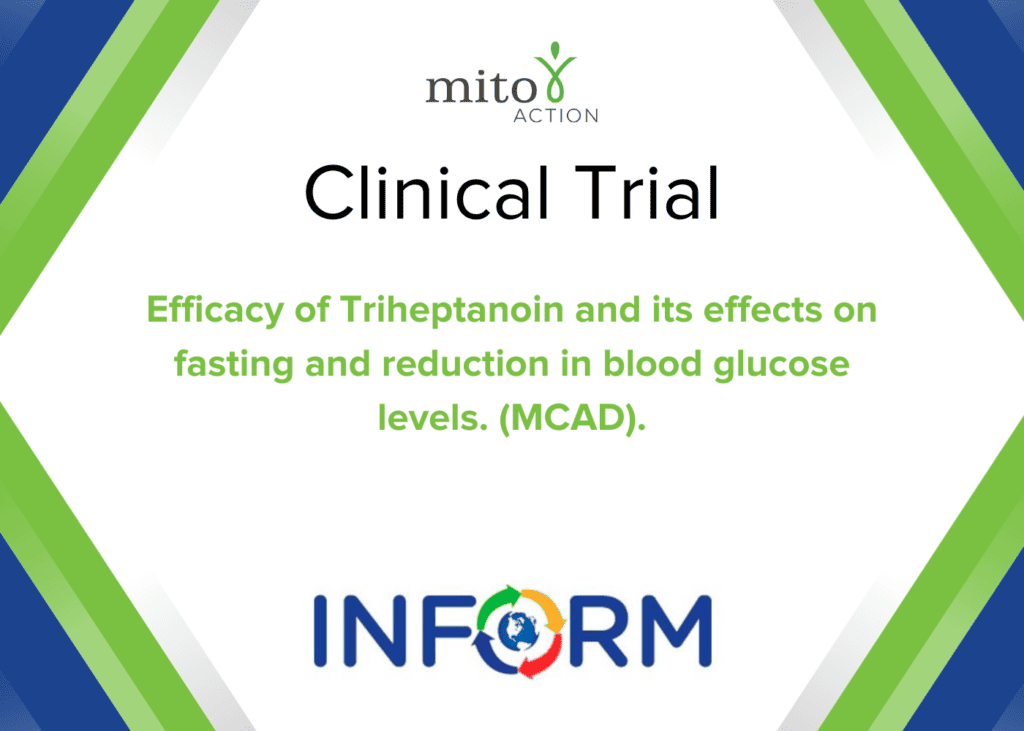
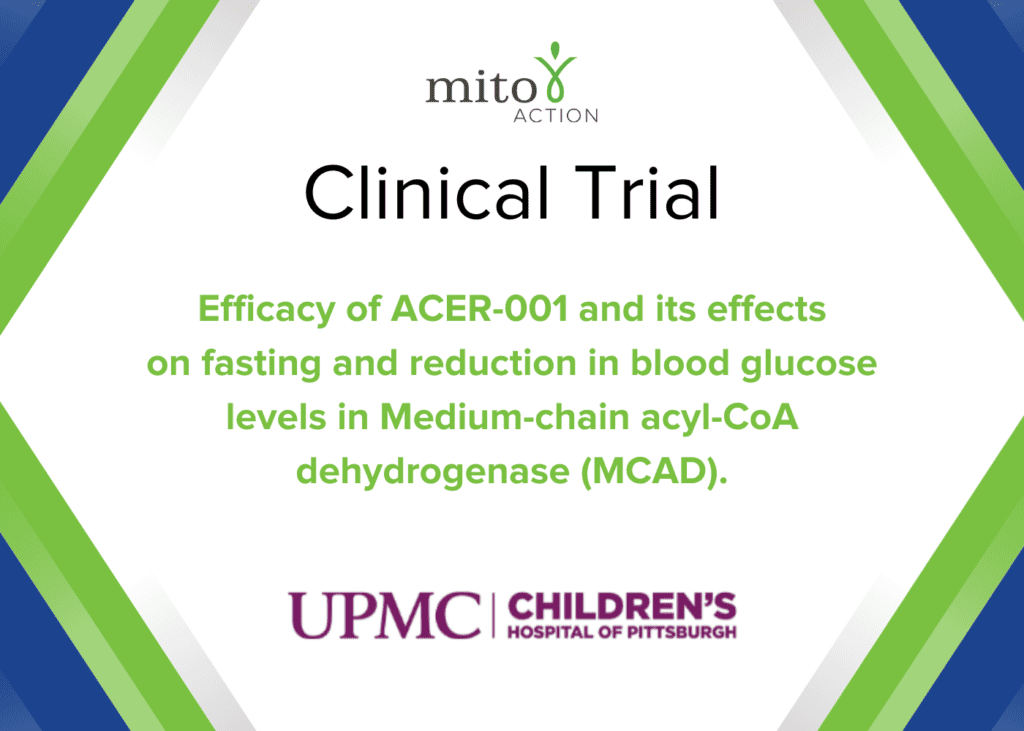
Current Clinical Trials